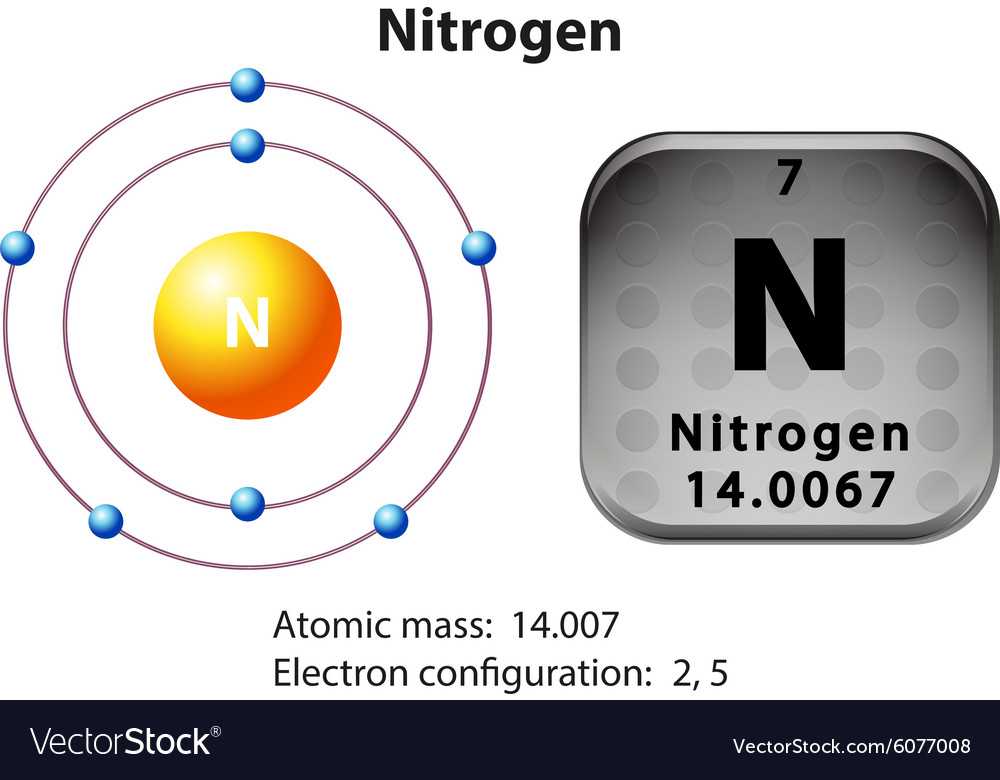
Symbol and electron diagram for nitrogen Vector Image
Campaigning & outreach Journals, books & databases News & events Locations & contacts Element Nitrogen (N), Group 15, Atomic Number 7, p-block, Mass 14.007. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.

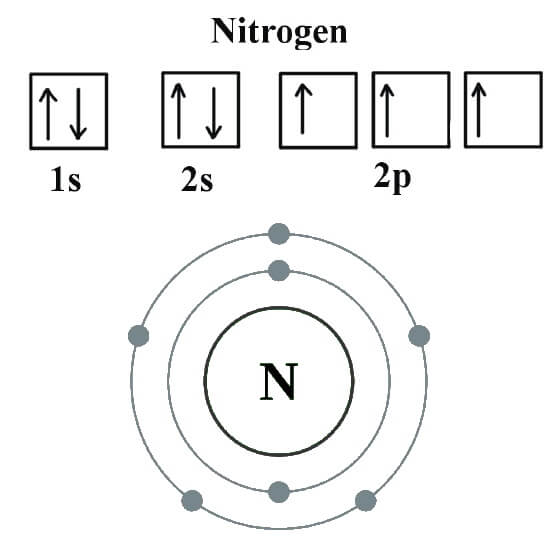
Orbital Diagram For Nitrogen (N) Nitrogen Electron Configuration
The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry.. The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, we have no choice. One.

Nitrogen Facts, Symbol, Discovery, Properties, Uses
Figure 4.8: Electron arrangement of a fluorine atom. Neon. Neon (\(\text{Ne}\)) has an atomic number of 10, meaning that a neutral atom also has 10 electrons. The first 2 electrons are found in the first energy level and the last 8 are found in the second energy level. . Figure 4.9: Electron arrangement of a neon atom.

Diagram representation of the element nitrogen Vector Image
This electron arrangement indicates that the outermost orbit of Nitrogen (N) has 5 electrons. Hence, it lies in group 15. Why is Nitrogen in Period 2? Let me ask you a question. How many shells does Nitrogen have? It's 2. Right? You have already seen the bohr model of nitrogen element in the above table.
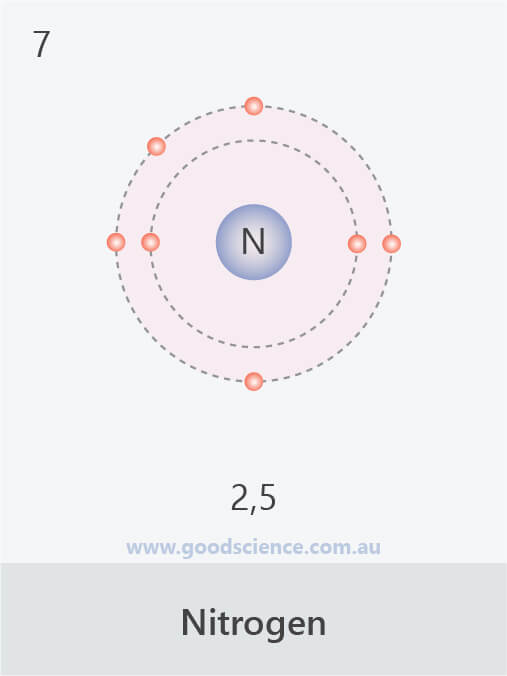
Electron Configuration (Elements 120) Good Science
In the quantum-mechanical model of an atom, electrons in the same atom that have the same principal quantum number (n) or principal energy level are said to occupy an electron shell of the atom. Orbitals define regions in space where you are likely to find electrons. As shown in Figure 3.7.2 3.7. 2 s orbitals are spherical shaped, and p.

Nitrogen Electron Configuration (N) with Orbital Diagram
The arrangement of electrons in nitrogen in specific rules in different orbits and orbitals is called the electron configuration of nitrogen. The electron configuration of nitrogen is [ He] 2s 2 2p 3 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.

Nitrogen, atomic structure Stock Image C018/3688 Science Photo Library
Nitrogen can also form ions by gaining or losing electrons. The Lewis dot structure of a nitrogen ion depends on the charge it carries. For example, the nitrogen ion with a positive charge (N+) has lost one electron and is represented by nitrogen's atomic symbol with a positive sign.On the other hand, the nitrogen ion with a negative charge (N-) has gained one electron, and its Lewis dot.
Electron Configuration for Nitrogen (N and N3 ion)
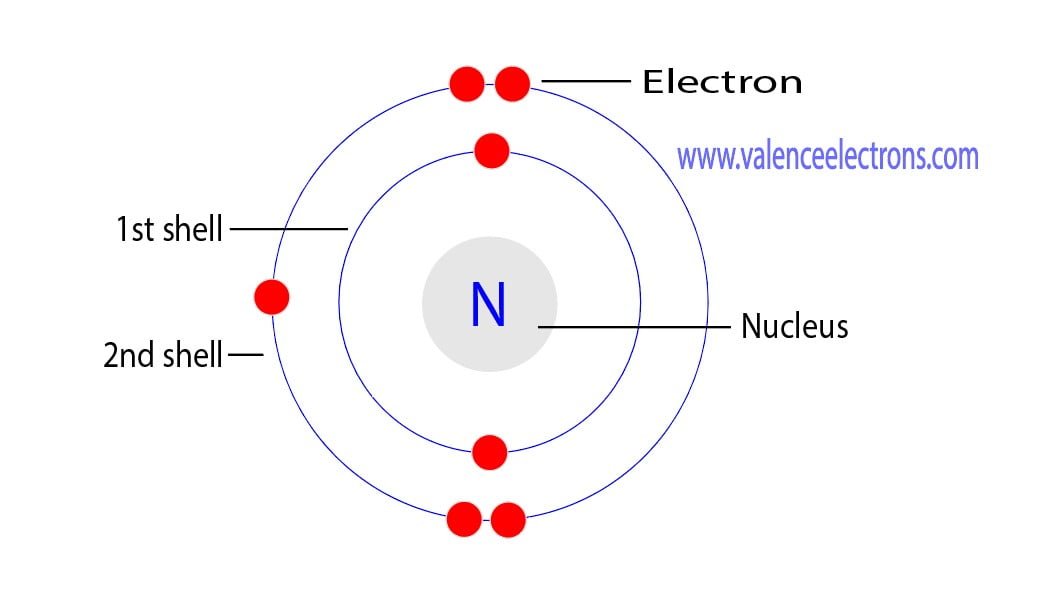
2.5: Arrangement of Electron (Shell Model) An electron shell is the outside part of an atom around the atomic nucleus. It is a group of atomic orbitals with the same value of the principal quantum number n n. Electron shells have one or more electron subshells, or sublevels. The name for electron shells comes from the Bohr model, in which.
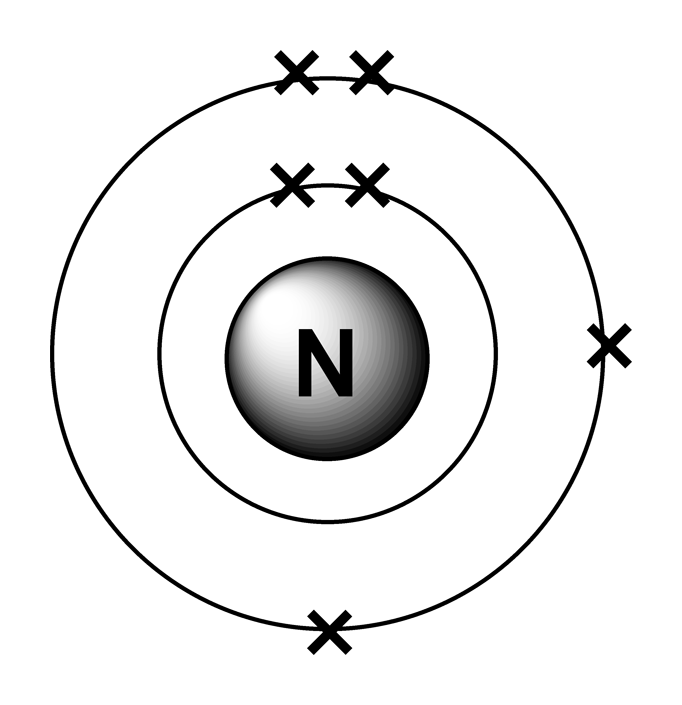
Electron arrangements
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating.
How many valence electrons does nitrogen have? Ask4Essay
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,
/nitrogenaton-58b602813df78cdcd83d8348.jpg)
Atoms Diagrams Electron Configurations of Elements
March 23, 2023 by Jay Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Free Gift for you: Interactive Periodic Table

How to write the Electronic Configuration of Nitrogen Chemical
1 to 20: Predicting an electron arrangement The electron arrangement of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. Sodium atoms have 11.
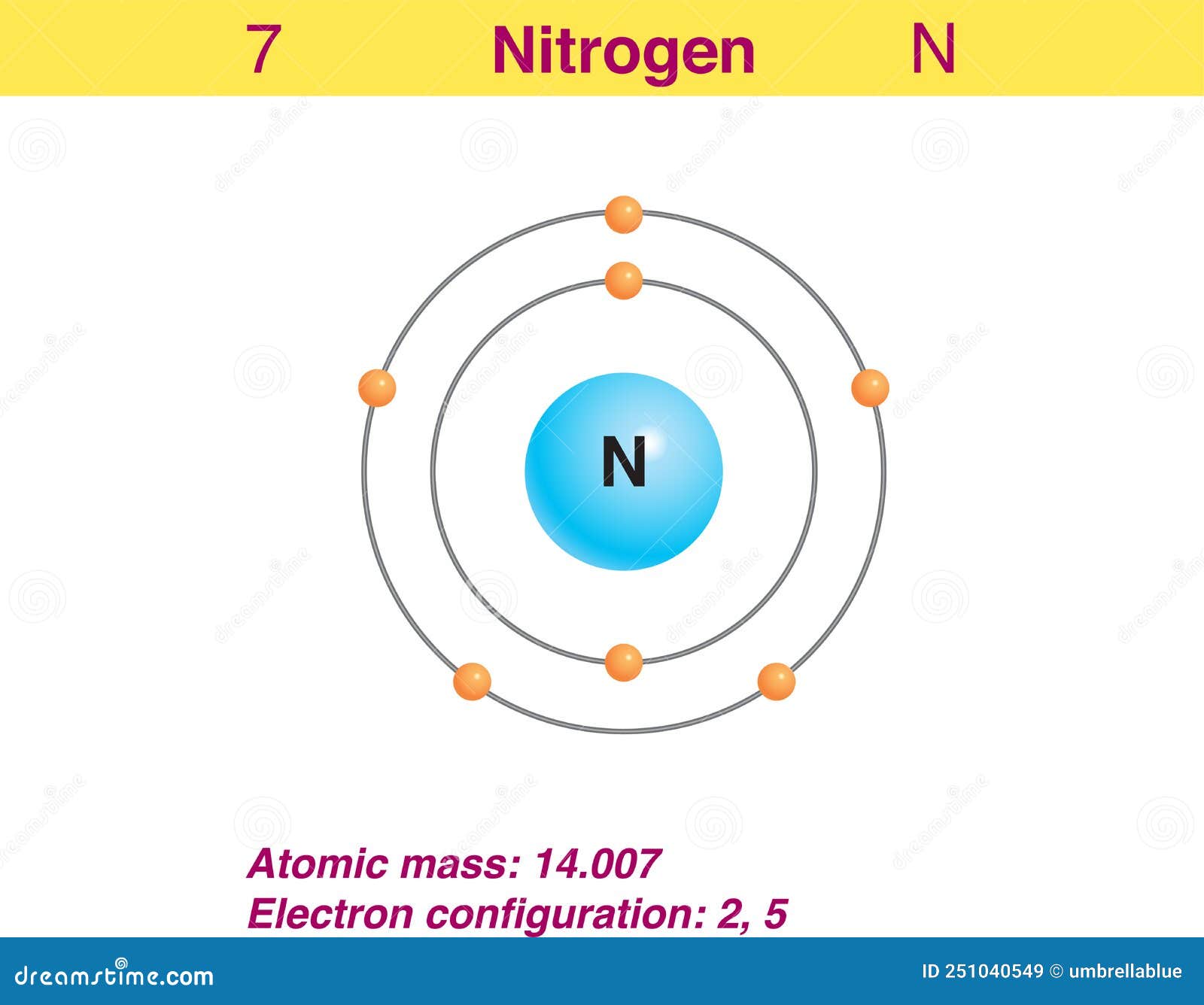
Electron of the Element Nitrogen Stock Vector Illustration of print
A Lewis Structure is a depiction of the arrangement of electrons in the standalone atoms of an element. In the Lewis Structure, electrons are depicted as dots.. Nitrogen's valence electron count, however, is 5, owing to its position in the 5th group of the periodic table. The plus sign denotes the absence of 1 electron; therefore, it is.

3d render of atom structure of nitrogen isolated over white background
The full electron configuration for nitrogen is "1s"^ 2"2s"^2"2p"^3. The noble gas shorthand electron configuration is ["He"]"2s"^2"2p"^3". The atomic number of nitrogen is 7. This is the number of protons in the nuclei of nitrogen atoms. A neutral atom has the same number of electrons as protons. So the electron configuration will include 7 electrons placed into the appropriate s and p.

Bohr model Description & Development Britannica
Answers. Electrons are organized into shells and subshells around nuclei. The electron configuration states the arrangement of electrons in shells and subshells. Valence electrons are in the highest-numbered shell; all other electrons are core electrons.

Nitrogen Wikipedia
The electron configuration of nitrogen is 1s 2 2s 2 2p 3, helps to express how the electrons are arranged in nitrogen's atomic orbital.Let us study its electron configuration in detail. Nitrogen, a non-metal is found to be the lightest element of group 15 of periodic table.It is the 4 th highest electronegative element. It is a p-block element.